Medical Device Development
We have the experience and expertise to help you develop your medical device.
Medical devices are subject to regulatory requirements that ensure a well-documented and reviewable design process — and ultimately, a safe product. At Simplexity, our core design philosophy is that the best solution is the simplest one to reliably accomplish your goals. This guiding principle is particularly relevant in the medical device space, where reliability, safety, and intuitive design are of the utmost importance. Our ISO 13485:2016 certified compliant quality management system ensures consistent design and development of devices that are safe for their intended use.
Simplexity's Priorities in Design
As product realization experts, our priority is to work collaboratively with our clients to meet both the technical and business needs of new products. Our expert team focuses on project priorities such as:
- Intuitive, safe, and reliable operation
- Product performance
- Design for manufacturability (DFM)
- Device and consumable cost
- Risk management
- Development cost
- Speed to market
Simplexity’s Capabilities and Medical Device Development Expertise
We are a quality-driven engineering design company, and one way that we ensure high quality results is to bring together an exceptional team of engineers, project managers, and technicians. Our medical device development capabilities and expertise include:
- Medical device development and design to meet requirements for user needs, reliability, manufacturability, and performance
- Redesign of cleared or approved medical devices to improve manufacturability, reduce cost, or replace obsolete components
- Generation of design history file (DHF) documentation to fulfill regulatory submission requirements
- Identification and management of risk throughout the product development process
- Controlled builds of pre-production devices for design verification and usability studies
- Design verification testing
- Supplier evaluation and selection
- Design transfer to contract manufacturers
Our product engineers and project managers have worked with clients to design medical devices in the following product categories:
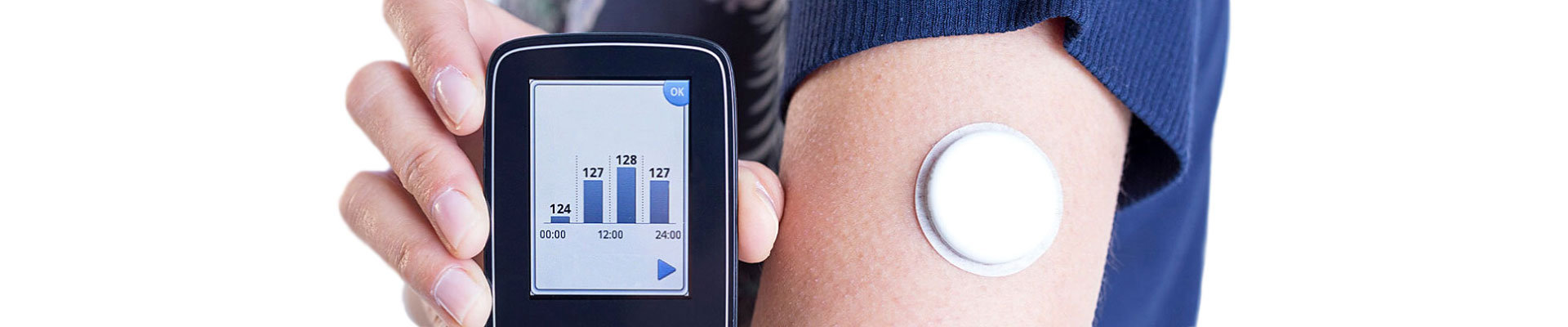
- Medical wearables and patient monitoring devices
- Surgical tools
- Drug delivery systems
- Endoscopy and catheter-based devices
- Home health devices
- Low-power wireless communications
Simplexity has a deep understanding of the medical device development space and the unique requirements that must be met to achieve success. We look forward to partnering with your company’s core technology experts, working shoulder-to-shoulder to enable the rapid transformation of novel concepts into real, safe products.
View Medical Device Case Studies

Reperio Health
Reperio Health
Learn More
CASE STUDY: OREGON HEALTH & SCIENCE UNIVERSITY (OHSU) VASCULAR MONITORING
CASE STUDY: OREGON HEALTH & SCIENCE UNIVERSITY (OHSU) VASCULAR MONITORING
Learn More
Bio1 Systems
Bio1 Systems
Learn More
Catheter Design Applications
Catheter Design Applications
Learn More
STERIFRE AURA
STERIFRE AURA
Sterifre Medical chose Simplexity to improve AURA, their automated point-of-care disinfection device. Noise reduction, weight reduction, and cost reduction were key in the improvement of this device while maintaining functionality and quality.
Learn More
Light Sensor Calibration System
Light Sensor Calibration System
Learn More
Computer aided CPR Training
Computer aided CPR Training
Working in close collaboration with HSI, Simplexity developed a microprocessor based LOOP CPR Controller that is used with a standard CPR manikin during training.
Learn More
Touch Spot
Touch Spot
Increasing patient compliance via an in-home, easy-to-use dried blood spot collection device
Learn More