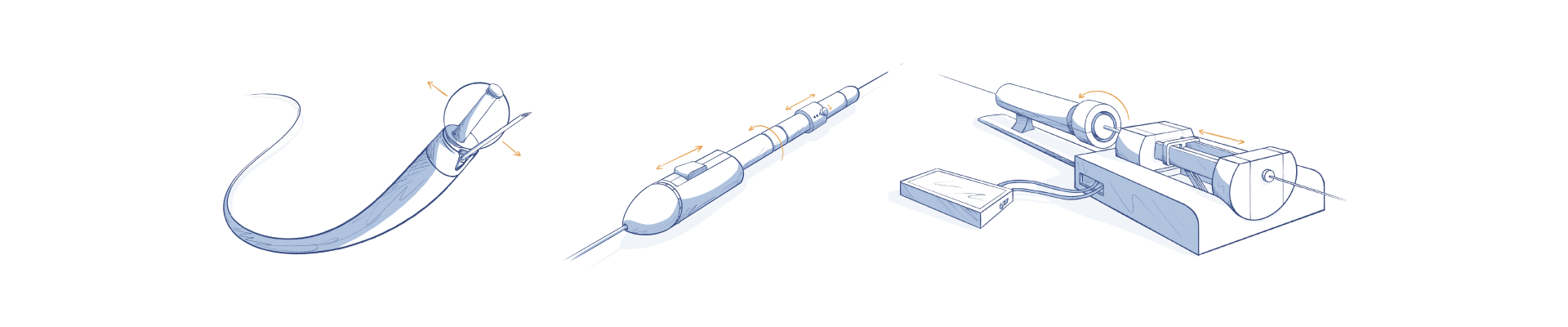
CASE STUDY: CATHETER DESIGN APPLICATIONS
Simplexity has engineered many advanced catheter designs for a variety of applications that can be used inside the body. This depth of experience in the medical device market, our mechanical, electrical, and systems expertise, and ISO 13485 certification for medical device product development give our clients confidence in the quality, precision, performance and integrity of our design engineering work in this space.
Learn more below on applications experience in the areas of catheter tip, handle and systems design.
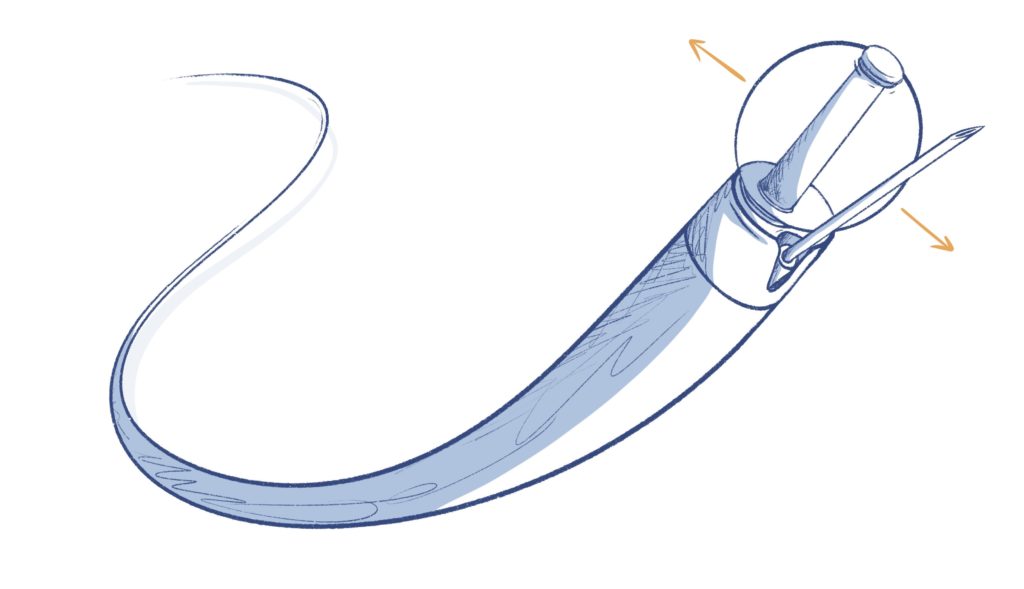
CATHETER TIP DESIGN
- Varied applications inside the body (lung, GI tract, or blood vessels)
- May have mechanical (balloons, needles, other tools), electrical (cauterizing, ultrasound, etc.) that interact with the body
- May also deploy a device (like a stent or staples) that are left in the body
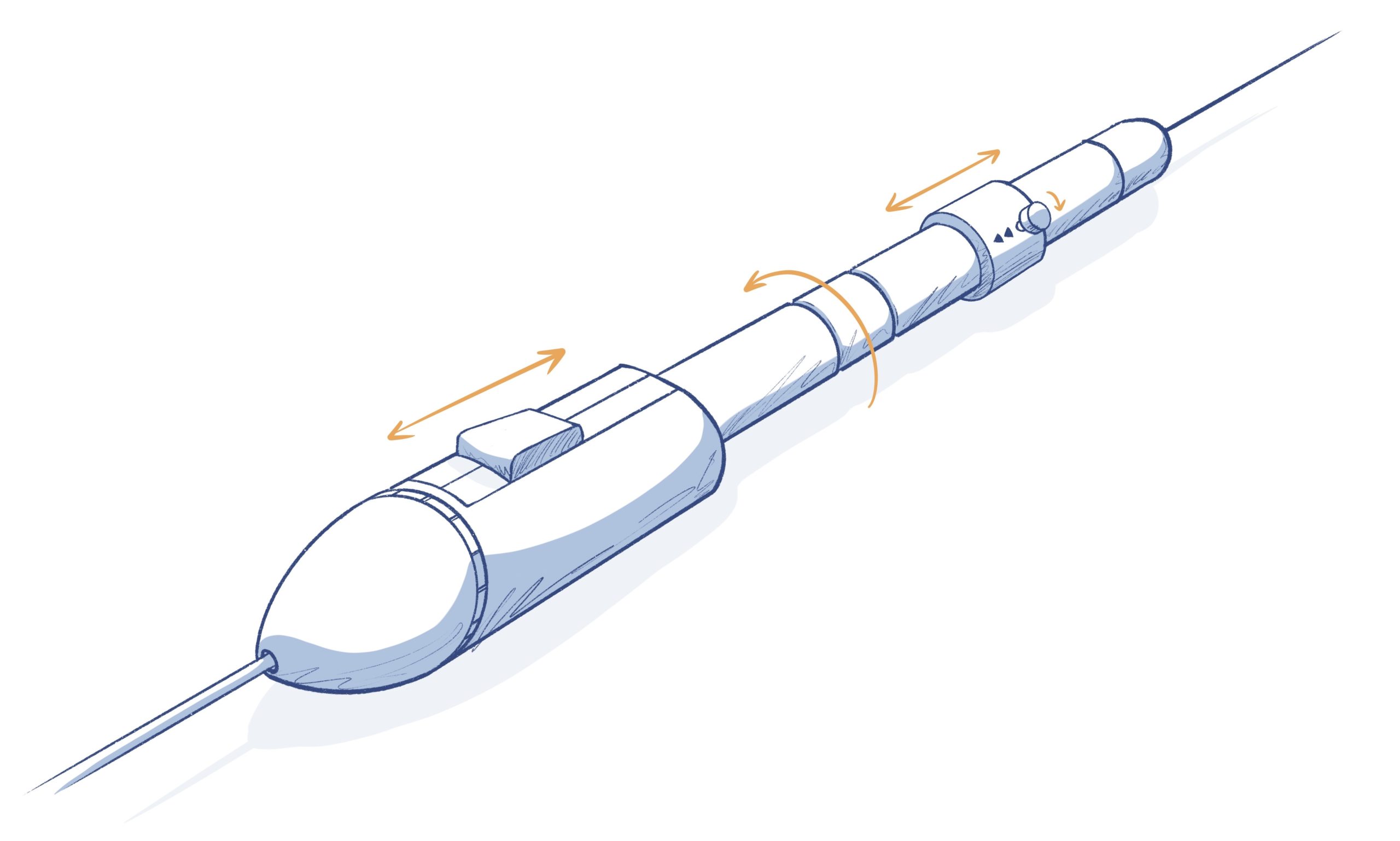
CATHETER HANDLE DESIGN
- The handle often has knobs, slides, levers, etc. to manipulate the catheter inside the body
- Often operated manually by a physician, but may have other systems that control mechanical features, fluidics, electronics, etc.
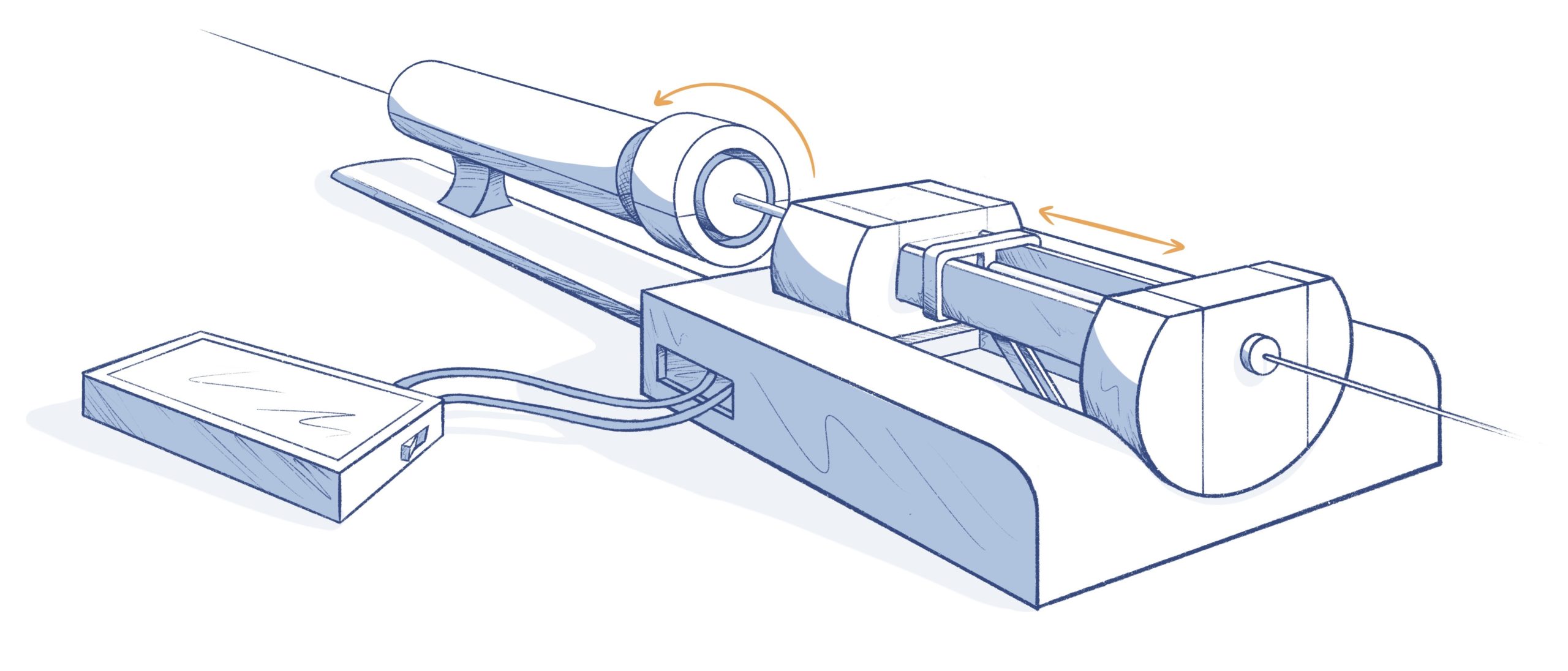
CATHETER CONTROL SYSTEMS DESIGN
- Some control systems are fairly simple, mainly for mechanical control or fluidics for flushing saline or contrast agents into the catheter
- Basic user interfaces – buttons, a simple touchscreen, or a computer application
Catheter devices are subject to regulatory requirements that ensure a well-documented and reviewable design process to provide a safe product that can be used in the medical device space, where reliability, safety, and intuitive design are of the utmost importance. Our ISO 13485:2016 certified compliant quality management system ensures consistent design and development of devices that are safe for their intended use and can meet and exceed our client’s product development, quality, and performance standards.