CASE STUDY: OSHERU ZIPLYFT SURGICAL DEVICE
Medical Procedure Enhancement Through Innovative Product Development
The Challenge
Osheru, a medical device company started by two Oregon-based surgeons had created a patented, first-generation Ziplyft prototype for improving blepharoplasty surgery, a procedure for removing excess tissue in the upper eyelid. Blepharoplasty is a standard medical procedure performed by ophthalmologists and plastic surgeons, but Osheru saw a way with Ziplyft to improve the physician experience in performing the procedure, the time the procedure took, the consistency of the results, as well as patient recovery times and outcomes.
While Osheru’s initial testing of the Ziplyft device had shown promise, clamping and cutting performance of the first generation device in clinical evaluations were below target goals. Osheru approached Simplexity for help with redesigning Ziplyft to improve the strength and uniformity of clamping while enabling a smoother cutting action, better satisfying needs of physician and patient.
Simplexity’s scope of work included engineering evaluation of the initial design for both performance and cost, testing and performance characterization of Ziplyft clamping and cutting functions, recommendations for design & assembly improvements and implementation of those changes in a Generation 2 design of the device. Upon Gen 2 design release, Simplexity was also asked to support small quantity device assembly builds for Osheru clinical testing, followed with NPI (New Product Introduction) support for a Pilot build of 450 devices for expanded surgeon initial use in the operating room instead of testing.
Blepharoplasty Described
Dr. Patricia Buehler' s Story
Dr. Patricia Buehler has been an eye surgeon for over 25 years and has performed thousands of blepharoplasties or eyelid lifts. This procedure restores peripheral vision and creates a younger more revitalized appearance by removing excess skin from the upper lid. Her innovation insight was inspired by an ancient Egyptian medicine exhibit showing how Egyptian healers removed excess lid skin by compressing the skin between two small twigs, depriving it of blood supply. The excess skin fell off within 5 days.
Impressed by this compelling idea, she designed and patented the Ziplyft, a revolutionary device that enables the first minimally invasive surgery for lids.
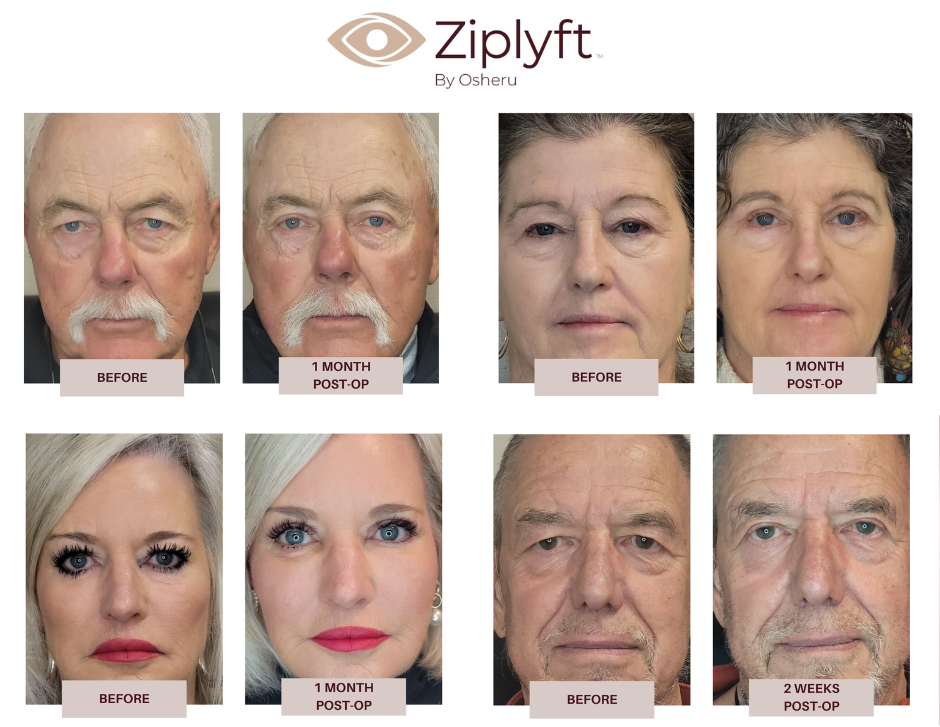
Blepharoplasty Surgery Results
We’re grateful to the Simplexity team for helping us improve the design and assembly of the Ziplyft and making the Gen 2 devices available for clinical use. Thanks to this progress, we’ve now completed over 100 blepharoplasty procedures and trained 9 surgeons with the Ziplyft device.
~ Dr. Patricia Buehler, Founder and CEO, Osheru
The Solution
Ziplyft establishes a new category in aesthetic medicine through its compressive skin contouring (CoCo) technique-a paradigm-shifting technique that achieves bloodless, sutureless skin excision through controlled mechanical compression. Unlike traditional surgical approaches that rely on cautery and suturing, CoCo procedures reduce surgical trauma, eliminates suture-related complications, shorten procedure times, and enhance patient recovery.
As the first device in this emerging category, Ziplyft redefines how physicians approach skin excision and rejuvenation. The CoCo technique has broad potential applications beyond the upper eyelid, including the lower eyelid, brow, jowl, chin, and underarm, opening the door to an entirely new class of minimally invasive aesthetic treatments.
Osheru’s Gen 1 prototype showed promise by using the Coco technique, but exhibited low eyelid tissue clamping force (poor hemostasis) and difficult tissue excision (incomplete cutting) in clinical testing. The first step Simplexity took to help was to define engineering metrics and test methods for quantifying performance against requirements. Simplexity then established testing criteria, designed test fixtures and conducted characterization testing of the protype to understand where to modify the design. As design iterations were proposed, they were then evaluated on the same test fixtures to verify the improved performance before design release. Design history file documentation for the Class 1 device was supported by Simplexity’s Quality Engineering team within the client-managed Quality Management System.
During the re-design for performance improvements, Simplexity also conducted a Design for Manufacturing review to improve overall device assembly processes, reducing COGS (Cost of Goods Sold) of the device and ensuring repeatable device performance in volume. Assembly fixtures and aids were developed and refined in low-volume device builds at Simplexity, and applied to a larger pilot device build (450 devices) at a partner contract manufacturer (CM) with Simplexity’s new product introduction (NPI) support for assembly setup, build execution and engineering verification testing.
Mechanical Engineering
Initial mechanical evaluation of the Ziplyft prototype yielded several areas where components and part interfaces could be redesigned for smoother device operation. This also resulted in more consistent application of tissue clamp force across the patient eyelid, as well as optimization of total pressure applied.
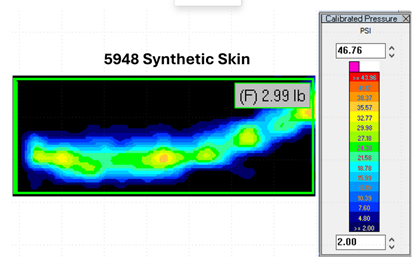
Simplexity captured quantitative data of how much clamp force was being applied to the eyelid by putting pressure sensitive film into the device using synthetic skin. This allowed the team to calculate how much force would be needed to create a seal, which could then be fed back into the engineering model. From that, the size and materials of the components could be increased to meet the target pressures.
Capturing quantitative device clamp force/pressure data for comparative design evaluation
Pressure-Sensitive Film
Tekscan Pressure Sensing
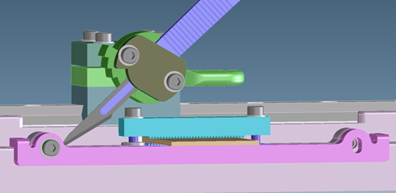
Cutting performance was addressed by modifying the design to better align blade sliding surfaces, as well as replacing the prototype cutting blade with an improved blade geometry.
CAD view of the cutting fixture used to assess blade cut performance and optimal blade design
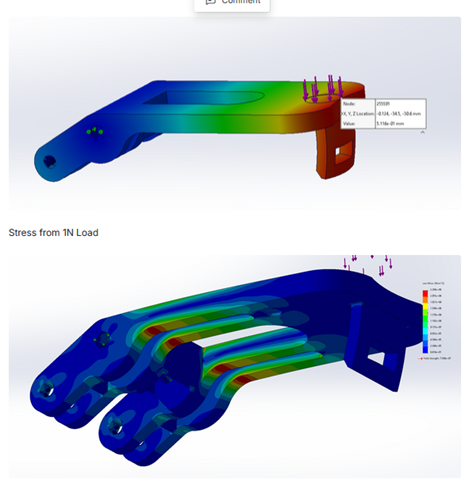
To enable improved device performance, Simplexity investigated several material options for key components of the device. This required a balance of effective part design and material selection, aided by Finite Element Analysis and physical part testing.
Finite Element Analysis and material compatibility evaluation and recommendations – coefficient of friction (COF) vs strength & sterilization
Simplexity was a valuable partner to Osheru when we needed to improve performance in our Gen 1 Ziplyft devices. They followed a rigorous process to evaluate and quantify the cutting motion and clamping force, which they then applied to the design of the Gen 2 device. Simplexity also partnered very effectively with our Contract Manufacturer in transitioning the design to a Pilot build of several hundred devices.
~ Dr. Knute Buehler, Founder and COO, Osheru
New Product Introduction (NPI) and Manufacturing
With the Generation 2 design of the device defined, Simplexity was asked to support planning, preparation and support for a Pilot-quantity assembly build of the device (~450 devices) at a partner contract manufacturer site. This would enable Osheru to extend their clinical evaluation of the new design to more doctors and patients, providing valuable feedback to Osheru for ongoing device development.
Simplexity’s role in supporting the Pilot assembly build included:
- Supplier identification, qualification and support for component parts
- Management of injection mold tooling at both domestic and off-shore suppliers
- Assembly and test fixture design, build and qualification
- On-site engagement with the production-level contract manufacturer for pilot device assembly
- Quality engineering planning & support for ensuring device build quality and execution of engineering verification test protocols
Simplexity was instrumental in helping Osheru manage device tooling and sourced components throughout Gen2 development. Their project management, communication tools and knowledge of supply chain and manufacturing processes were integral to our success in building hundreds of Gen2 devices for product validation testing and clinical training.
~ Alyssa Pineau, Project Manager, Osheru
The Results
With the help of Simplexity’s expertise in product design, testing and manufacturing, Osheru was able to improve performance of their initial device design, and extend device use to a larger set of physician users. Higher clamp force and consistent cut performance in the device helped surgeons perform more efficient surgeries and ensured better case outcomes for patients undergoing blepharoplasty procedure.
Osheru has completed a pilot study with 5 surgeons doing 30 Ziplyft blepharoplasties. The preliminary results showed significantly shorter case times for the procedure averaging 12 minutes per case when traditional blepharoplasty can take 30-60 minutes. In 30 of the 30 cases, surgeons reported that they believed the Ziplyft cases had less bruising than their traditional blepharoplasty cases. Patient satisfaction with the procedure was high.
- For Osheru as a client: acceleration of design iterations & testing, improved design & product cost structure
- For Surgeons: a more efficient and elegant procedure in less than half the time, with improved symmetry and without suture related complications
Discover how Dr. Ristvedt is transforming eyelid surgery with the innovative Ziplyft device!
- For the Patient: better outcomes, shorter procedure and recovery times
Revolutionary New Device and Technique for Upper Lid Surgery
Resources
- State of MedTech Podcast: Eyelid Rejuvenation via Minimally Invasive Lid Surgery with Patricia Buehler, MD, Founder of Osheru
- Cascade Business News: Bend Company Develops Innovative Medical Device
- Cascade Business News: Dr. Patricia Buehler Wins People’s Choice Award for her Innovative New Device for Eyelid Surgery