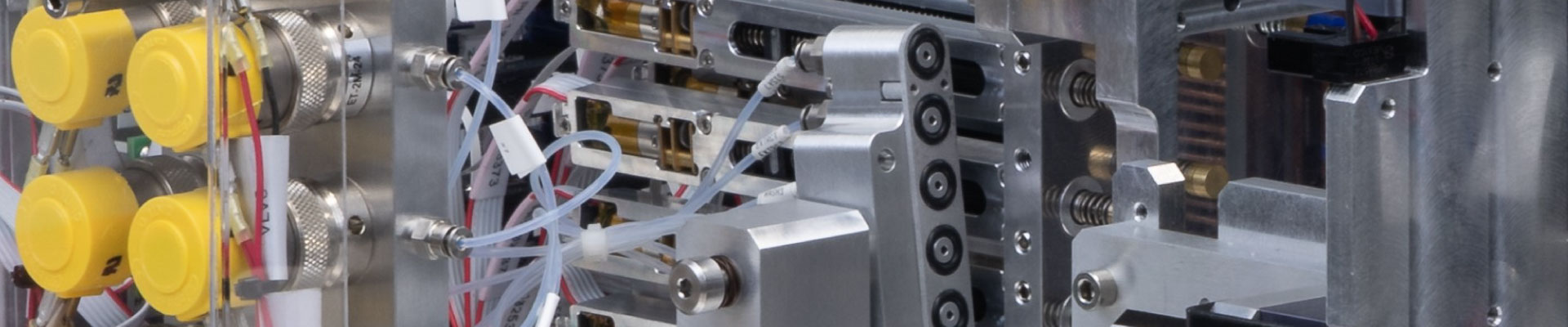
CASE STUDY: MOLECULAR DIAGNOSTIC TESTBED
In molecular diagnostics development and validation, assays are optimized to generate results on a variety of samples. A client approached Simplexity with the idea to develop a testbed enabling end-to-end assay workflow development on the prototype cartridge for a point-of-care molecular diagnostic device.
The Product
- The primary goal of this testbed is to quickly enable end-to-end assay workflow development on the prototype cartridge for a POC molecular diagnostic device
- The testbed is also used to assess the feasibility of the instrument product architecture
- The cartridge has typical interface features such as precise optical alignment, microfluidic controls, thermocycling, sonication, and paramagnetic particles (PMP) isolation
Our Role
- Delivered a fully functional testbed which interfaces with the prototype cartridge to run the assay workflow end-to-end via software control in 12 weeks
- Worked closely with the cartridge team to analyze, design, and lock the cartridge-to-instrument interfaces in 2 weeks
- Delivered test software enabling assay scientists to easily program assay development workflows
- The testbed architecture enabled the cartridge team to quickly iterate design changes
- Testbed provides a preliminary product architecture for the production instrument design