COVID-19 Product Development
As an ISO-13485 certified product development company specializing in solutions to complex problems, Simplexity is here to assist in the fight against COVID-19. Whether it’s designing a molecular diagnostics solution or a medical device , our experienced product development engineers are ready to help you bring your lifesaving product to market in the fastest, safest, and most reliable way possible.
West-Coast Locations Available Worldwide
Simplexity is fully operational during the COVID-19 pandemic with four West-Coast office locations available to serve you both locally and worldwide. We offer a full range of services to support your molecular diagnostics and medical device needs including design, analysis, engineering, prototyping, testing, and all aspects of the product development process.
Quality Engineering for COVID-19 Product Development
Project support is available to your team with a dedicated quality-driven team of engineers, project managers, and technicians that can take your product to market faster. Diagnostic instruments require a unique blend of systems design expertise and biological collaboration experience that Simplexity can provide. Our ISO 13485:2016 certified quality management system ensures consistent design and development of devices that are safe for their intended use.
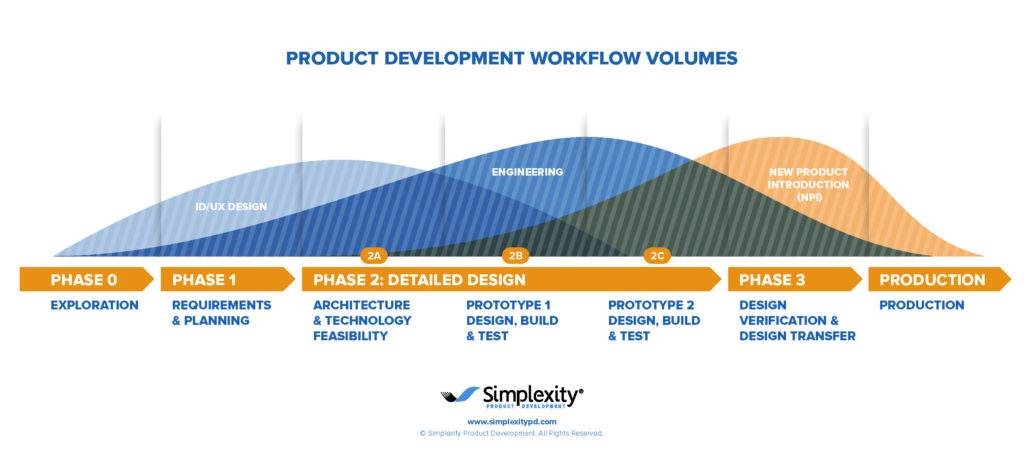
New Product Introduction Services
Simplexity's New Product Introduction (NPI) process is available to help you lower your product costs, reduce time to market, and manage product quality through all phases of the product development process.
Culture of Support
We are dedicated to our partners and the community. Simplexity employees have volunteered for multiple projects to combat the spread of this deadly disease and we will continue to offer our expertise to serve those in need until COVID-19 is no longer a threat to society.
Domain Knowledge
We are in the midst of developing diagnostic instruments with manufacturing and scientific partners that meet rigorous requirements and accelerated schedules. Our talented team is made up of engineers that specialize in technical disciplines applicable to any stage of a product’s development.
Grant Resources Available
Organizations such as The National Institutes of Health (NIH), BARDA, and The Department of Health and Human Services (HHS) have grants and partnerships available to help with access to capital to reach your goal. Financial support may be available to your organization through programs like the RADx Tech initiative that aim to speed the development, validation, and commercialization of innovative point-of-care, home-based, and clinical tests. Companies who seek or receive outside funding through these types of programs often select Simplexity as their engineering partner to have a full engineering team already in place.
Engineering a COVID-19-Related Molecular Diagnostic Product or Medical Device?
We can help!